Take the smart route to compliance with BSI’s Compliance Navigator
CEO? Drive efficiencies and maximize the ROI from your new product development
Make sure your whole business has a clear view of the regulatory landscape to get to market faster
You don’t want compliance risks slowing down your innovation pipeline. BSI’s Compliance Navigator helps your organization to pre-empt compliance issues faster than ever before.
Compliance Navigator
The smart, simple way to manage your medical device compliance.
Introduce smarter working to your company
100% relevance to your business
Save time, with device-specific profiles that allow your team to manage the standards and regulations that matter to your products.
Co-ordination across the business
Increase efficiency, minimize risk and reduce costs, with unlimited multi-user access to UK, US, European and internationally-adopted standards.

An authoritative library in one place
An intelligent search tool allows staff to quickly search over 4,000 essential documents including UK, US, European and internationally-adopted standards as well as regulations for medical devices.
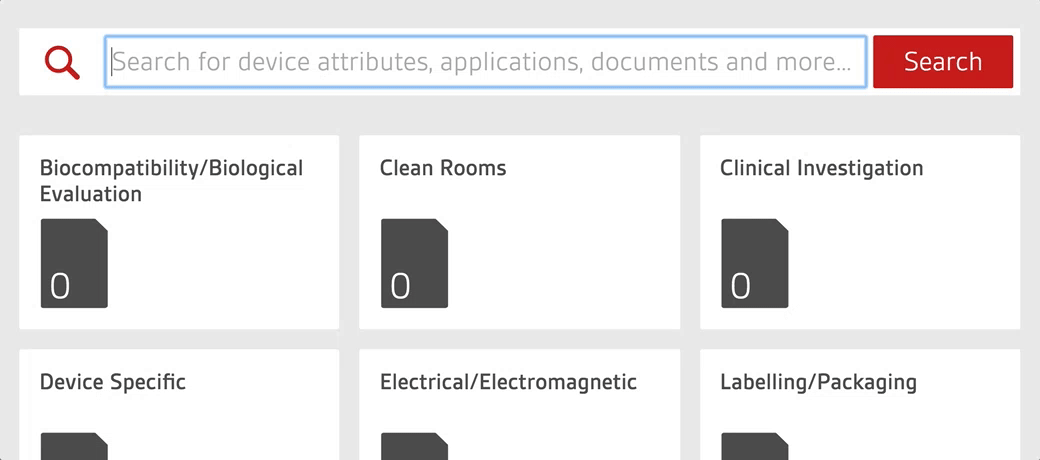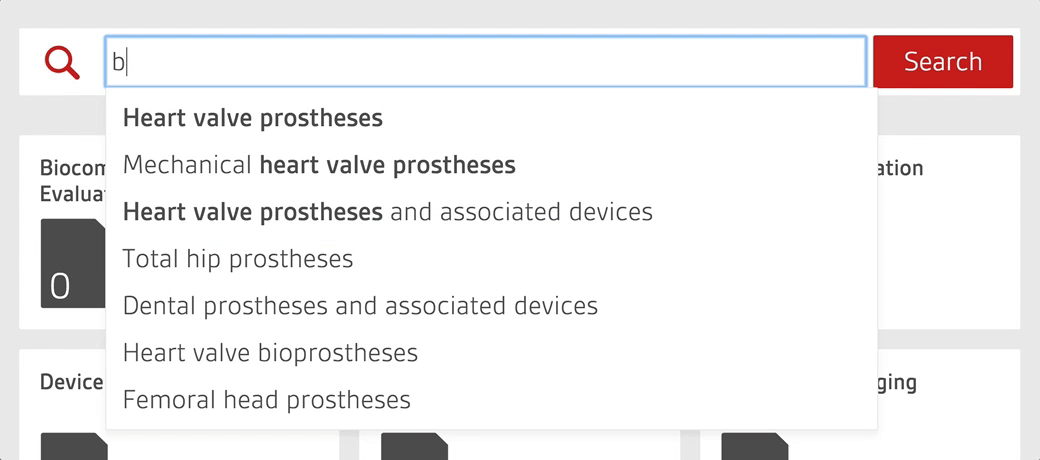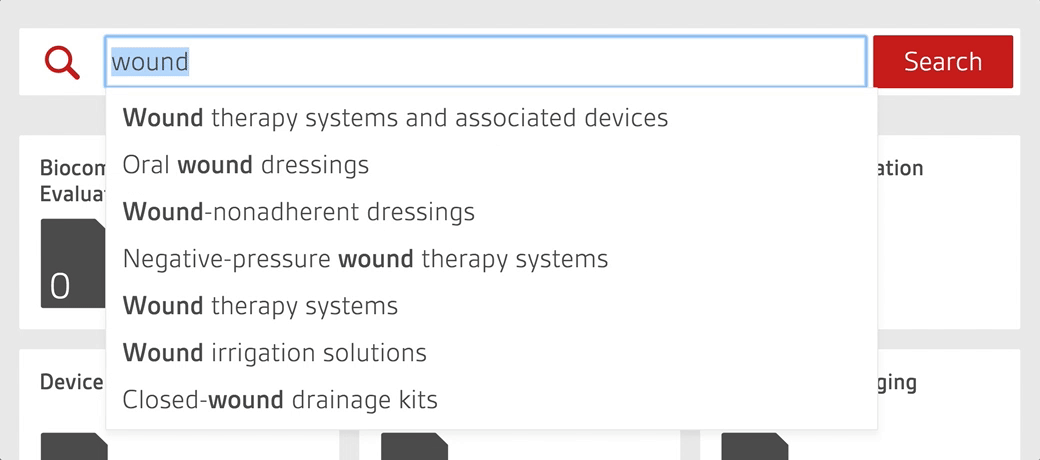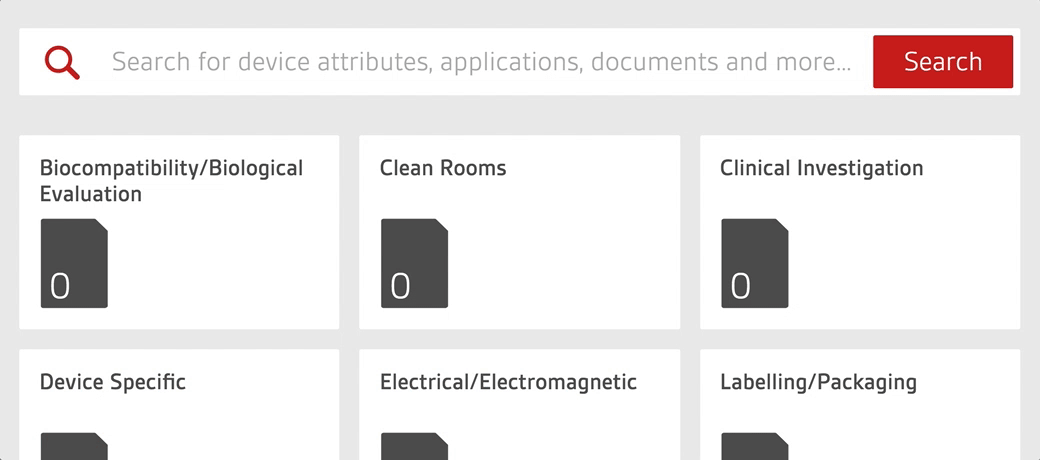
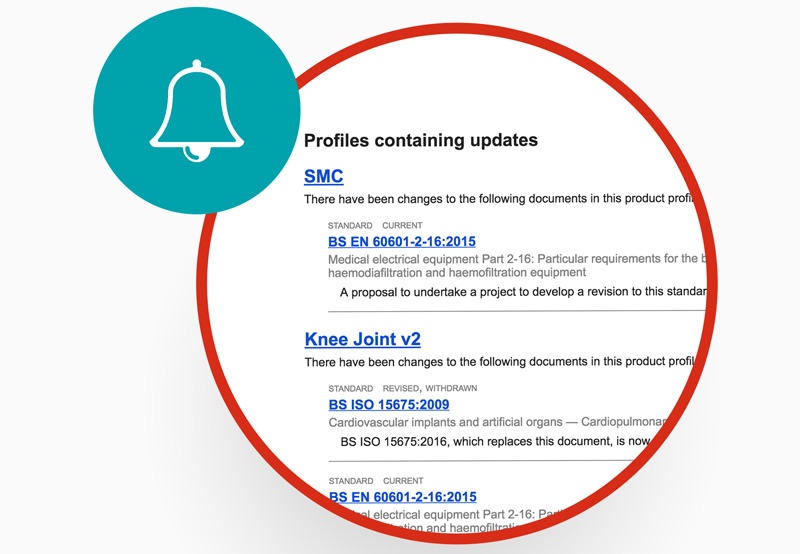
Alerts to changing standards
Put your team on the front foot, with alerts for when standards change and advance warnings for upcoming changes to BS standards.
Expert commentary and Smart Support
Reduce the costs of expensive external consultants. Expert context and guidance means that your team can interpret changes to new standards and the MD / IVD regulations quickly and accurately.

New changes shown at a glance
Drive efficiency with red-line change tracking, as staff can immediately spot changes to standards – saving hours of time spent comparing old and new.

An up-to-date digital library in one place
Access UK, US, European and internationally-adopted standards as well as regulations for medical devices.

A simple 3-step process for your business
Getting started is easy:
Create product profiles
Set alert notifications
Receive alerts and keep compliant
Plus
Easy set-up and great support
Learning to use Compliance Navigator is quick and easy, but if you need support, our customer service team provides your business with unlimited personalized training and tailored set-up, as well as knowledge tools such as tutorials and videos.
See how it works
What users love
A selection of testimonials from our clients.
“It’s not just the direct time saving of locating information quickly, it’s a much faster, accelerated workflow.”
“Easy to set up, understand and use. Being notified of upcoming changes is particularly useful…I can go quickly into the system and see how it will impact the product and our timelines.”

