Biomedical applications of 3D printing pt. 2
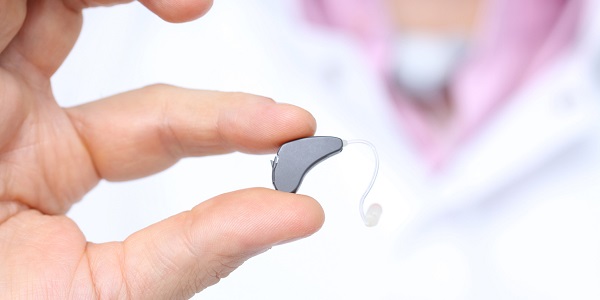
3D printing is used in the manufacture of dental devices, hearing aids, orthotics and prosthetics
Dental
Dentistry has in general been an early adopter of new technologies and has embraced 3D printing not only for guides, but also for a range of dental devices, most notably in a commercial sense for bridges and crowns. This has been associated with increasing availability of intraoral 3D scanning techniques. Generally, the devices are produced in cobalt chrome alloy or medical grade titanium–aluminium–vanadium alloy, or through producing sacrificial models which can be used as patterns for casting.
Hearing Aids
One area in which 3D printing techniques have almost completely displaced conventional manufacturing techniques is in the production of in-the-ear hearing aids. These are produced based on a laser scan of an impression of the inner ear, or on the basis of an intra-aural scan. From this a hollow shell is designed, with the internal space configured to accommodate the functional electronic hearing aid unit – in effect what is produced is packaging for the hearing aid which is a custom fit to the patient’s ear.
Orthotics
A range of foot and ankle-foot orthoses have been researched and brought to the market. These are designed to re-align or provide pain relief to patients with a damaged or diseased lower limb. The design is based on an external scan of the foot and lower leg, with correction applied for re-alignment or cushioning and with polymer 3D printing then used to create a device. The geometric freedom offered by 3D printing can allow for the mechanical properties of the devices to be locally tailored, resulting in an orthosis which is flexible and accommodating in some areas, but rigid and supportive in others.
Prosthetics
Prosthetic applications of 3D printing fall into two categories: (i) using 3D printing to produce externally applied prosthetics and (ii) the manufacture of polymer or metallic musculoskeletal implants using 3D printing.
3D printing of externally applied prostheses has two alternative strands. The first is the creation of bespoke covers for prosthetic devices, generally with the aim of making devices more aesthetically pleasing, allowing personalization of both geometry and design. The second approach is to use 3D printing techniques to make functional elements of the prosthetic, and a number of open source projects have emerged over the years, offering designs for patients and their carers to manufacture devices at home. 3D printed prosthetic hands for children, for example, have the advantage that designs can be upgraded as the children grow, or in response to changes in motor skills.
3D printed implants have attracted significant public attention and have shown significant growth over the past decade. Two main strategies have emerged, one focussed on custom devices, and the other on enhancing non-custom devices. Much of the original work focussed on custom implants, normally in medical grade titanium alloy, with implants for large bone defects and joint replacement the most common. The alternative approach is to exploit the ability of 3D printing to create complex porous geometries, but within a standardised product, an approach that Stryker has used in its Triathlon® Tritanium® Knee and the Tritanium® Posterior Lumbar Cage products. For custom joint replacement the production of revision implants can offer a more compelling rationale: revision implants are generally more complex and can have a greater need for customization.
This is an excerpt from the forthcoming white paper The impact and potential for 3D printing and bioprinting in the medical devices industry. To download our other medical device white papers, please visit the Insight page on the Compliance Navigator website.
Request more information today for a call back from a member of our sales team so that you can get a better understanding of how Compliance Navigator can meet your needs.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

