Artificial Intelligence (AI): The Regulatory Challenges That Lie Ahead

The use of AI and machine learning algorithms is growing rapidly across healthcare. Emerging solutions can potentially offer earlier diagnoses and select targeted treatments that will improve patient outcomes, whilst benefiting healthcare services by driving efficiency and effectiveness.
AI covers a broad range of technologies, involving complex tasks that would normally require human intervention. Machine learning is a subset of AI, and involves computers processing large amounts of data so that they can learn without being directly supervised.
For the purposes of this blog post, we have commonly referred to all these technologies and complex tasks as ‘AI’. However, it is recognized that this term covers a broad set of solutions (e.g. deep learning modules, neural networks) and that different approaches may well be needed across each subset, once definitive terminologies for each have been established.
The governance and regulation of AI solutions in healthcare have been identified as significant challenges. Studies have been initiated to consider how to address these, and BSI and AAMI have been exploring the role of standards to support the deployment of novel technologies across this highly regulated sector.
Both the UK and U.S. are acknowledged leaders in innovation for medical technologies and digital healthcare. The regulatory regimes in both countries are considered amongst the most rigorous and responsive to innovation worldwide. In addition, BSI and AAMI support regulators through the development and publication of national and international medical technology standards in their respective countries. These publications are used by industry to demonstrate conformity with regulatory frameworks across different countries and regions. A global approach to standardization of AI in healthcare is imperative to ensure its successful use in the future.
Current medical device regulations include software within their scope, depending on intended use. This can include software that is standalone or incorporated into an existing device. Standards covering the application of traditional Software As a Medical Device (SAMD) have been developed over recent years. However, AI solutions introduce a new set of challenges that have not been considered previously. These new challenges include the:
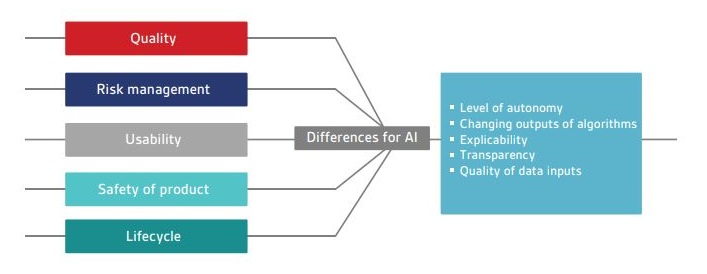
- level of autonomy introduced by AI technologies;
- ability of continuous learning systems to change their output over time in response to new data; and
- ability to explain and understand how an output has been reached.
Currently there are no standards that cover the definition, development, deployment and maintenance of AI in healthcare. Figure 1 outlines a selection of standards relating to traditional SAMD, along with the key differences that AI may introduce.
Figure 1 – Current areas for medical device software standards and differences that AI may introduce
Standardization can help address challenges relating to deployment of any new technology quickly and responsively. Standards can be developed to align with and support existing regulatory frameworks, whilst keeping pace with evolving technologies. Standardization as a global trading tool and as a means of promoting safety of products and their effectiveness is well established. Standards also represent consensus between all parties – industry, regulators and public interest. They help achieve consistency and harmonization across borders, through independent support of regulatory regimes. The independence of standards bodies, including BSI and AAMI, is a key component to the success of such aspirations and the agreements which underpin them.
This is an excerpt from BSI and AAMI's position paper: "The emergence of artificial intelligence and machine learning algorithms in healthcare: Recommendations to support governance and regulation”.
Request more information today for a call back from a member of our sales team so that you can get a better understanding of how Compliance Navigator can meet your needs.
The Compliance Navigator blog is issued for information only. It does not constitute an official or agreed position of BSI Standards Ltd or of the BSI Notified Body. The views expressed are entirely those of the authors.

