The Digital Revolution in Regulatory Document Management
The only digital platform giving you access to all the necessary international medical device standards and regulations to meet compliance
The only digital platform giving you access to all the necessary international medical device standards and regulations to meet compliance
The situation with regards to the Novel Coronavirus (COVID-19) outbreak is evolving rapidly. Where necessary, BSI is taking immediate and extensive actions to safeguard our people by managing the risk of infection within our operations and wherever we work for our clients. This action is in accordance with WHO and respective local government restrictions and/or prevention guidance.
Reduce Risk
Get advance warning of upcoming changes to BS medical device standards
Save Time
Create and export your own profiles and templates, with all relevant documents in one place.
Find Documents Fast
Search by device and keyword, filter and refine results to see every relevant standard and regulatory document in seconds
Miss Nothing
Be notified when changes happen and see exactly what’s changed at a glance
Get it Right
Interpret standards and regulatory information via independent Expert Commentary and Smart Support guides
There’s no room for error in medical device and IVD standards. You need all your team working with the same information, wherever they are. You need them to know when it’s going to change, to know the instant it changes and to know what those changes mean for your devices.
Compliance Navigator holds over 4,500 documents essential for medical device and IVD compliance. With multi-user access, it’s available to your whole team.
Put your team on the front foot, with alerts for when standards change and advance warnings for upcoming changes to BS standards.
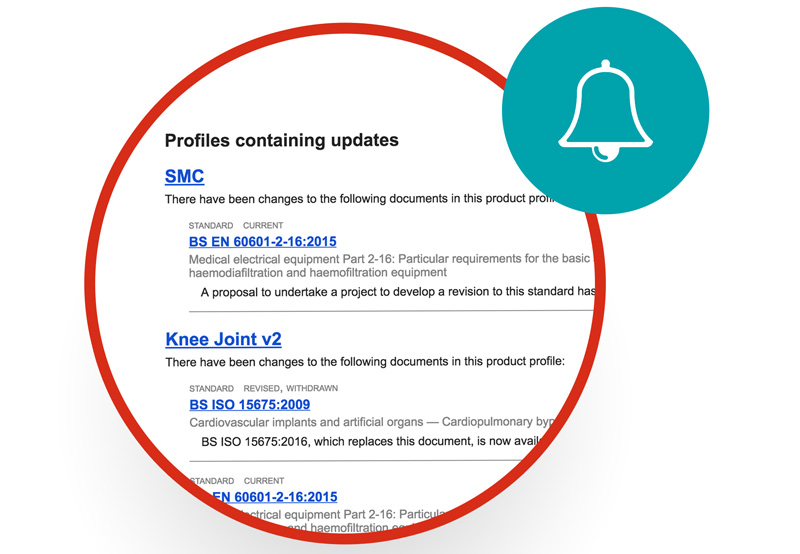
You can create regulatory profiles and templates, so you have the information you need in one place, along with links to relevant content outside of Compliance Navigator.

You can see the full text of standards, legislation and guidance, so you don’t miss anything important.
Full Medical Devices and IVD Regulations and guidance

You can prepare for the navigation to the MDR and IVDR with topic-by-topic Smart Support, with action points and practical guidance.

You’ll find it easy to see what’s new, with ‘red line’ changes between versions.

Use the most authoritative source of medical device standards information on the market. With over a century of experience, BSI is trusted by thousands of companies worldwide to help them stay compliant and grow their businesses.
“BSI is very professional… and consistent with the services provided.” Regulatory Affairs Manager, USA
Compliance Navigator holds over 4,000 BS and internationally recognized standards, including AAMI, CLSI and relevant ASTM standards, and relevant EU and US regulations including the MDR and IVDR, and guidance.
A selection of testimonials from our clients.
“It’s not just the direct time saving of locating information quickly, it’s a much faster, accelerated workflow.”
“Easy to set up, understand and use. Being notified of upcoming changes is particularly useful…I can go quickly into the system and see how it will impact the product and our timelines.”
Next steps
Take a tour to see how Compliance Navigator can help your business or contact us for a quotation.
